Vascularized TME model
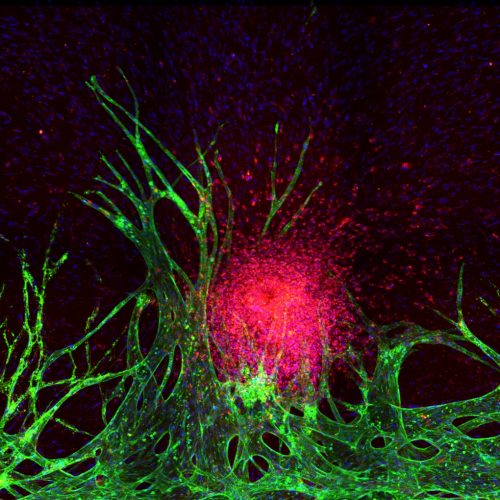
In cancer development, tumors induce angiogenesis, forming new blood vessels to supply nutrients, aid invasion, and enable metastasis. Vascularized tumors have a distinct tumor microenvironment (TME) critical for the efficacy of certain anticancer agents. Developing ex vivo vascularized TME models is crucial for drug development and personalized treatment. Our method uses a hydrogel matrix and microfluidic chip to encapsulate and pattern endothelial cells, fibroblasts, and cancer cells, inducing vasculogenesis and angiogenesis to form functional blood vessels. These models allow us to study differential drug responses, vascular changes, and patient-specific TME variations.
“Engineering tumor vasculature on Injection-Molded Plastic Array 3D Culture (IMPACT) platform”
Somin Lee, Jungeun Lim, James Yu, Jungho Ahn, Younggyun Lee, Noo Li Jeon, Lab Chip, 19, 2071-2080 (2019)
“Tumor spheroid-on-a-chip: a standardized microfluidic culture platform for investigating tumor angiogenesis”
Jihoon Ko, Jungho Ahn, Suryong Kim, Younggyun Lee, Jungseub Lee, Dohyun Park, Noo Li Jeon, Lab Chip, 19 (17), 2822-2833 (2019)
Anti-tumor immune response model
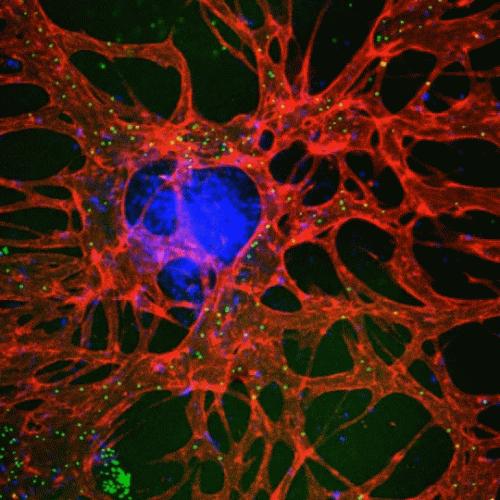
Modeling the anti-tumor immune response in vitro is crucial for understanding immune and cancer cell interactions within the complex tumor microenvironment (TME). Traditional 2D cell cultures are insufficient, but advanced platforms like organ-on-a-chip systems replicate 3D tissue conditions more accurately. Our lab’s CACI-IMPACT platform is a high-throughput 3D cytotoxicity assay that evaluates cytotoxic lymphocyte activity in a 3D extracellular matrix (ECM). Additionally, our vascularized 3D tumor model monitors natural killer (NK) cell activity against colorectal cancer cell lines. We aim to enhance these models to better predict immunotherapy outcomes and improve patient-specific treatments.
“Modeling 3D Human Tumor Lymphatic Vessel Network using High-throughput Platform”
Somin Lee, Habin Kang, Dohyun Park, James Yu, Seoung Kwon Koh, Duck Cho, Da-Hyun Kim, Kyung-Sun Kang, Noo Li Jeon, Advanced Biology, (2021)
“High-Throughput Microfluidic 3D Cytotoxicity Assay for Cancer Immunotherapy (CACI-IMPACT Platform)”
Dohyun Park, Kyungmin Son, Yunchan Hwang, Jihoon Ko, Younggyun Lee, Junsang Doh, Noo Li Jeon, Frontiers in Immunology, 10, 1133 (2019)
“High-Throughput 3D In Vitro Tumor Vasculature Model for Real-Time Monitoring of Immune Cell Infiltration and Cytotoxicity”
Jiyoung Song1, Hyeri Choi1, Seung Kwon Koh, Dohyun Park, James Yu, Habin Kang, Youngtaek Kim, Duck Cho, Noo Li Jeon, Frontiers in immunology, 3848 (2021)
Neurological disease model
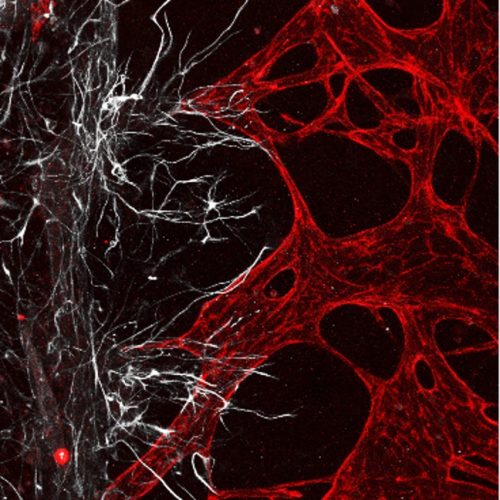
The development of three-dimensional neural networks is crucial for accurately replicating the in vivo environment, particularly for studying myelination, demyelination, and axonal growth in long-term cultures. Current 2D cultures and complex manufacturing processes are inadequate. Our lab has developed PDMS-based microfluidic chip models and a 3D-printed neuron-on-a-chip platform to better simulate neural environments. Our current focus is on developing a versatile peripheral-injury-on-a-chip platform, which facilitates the study of peripheral sensory neuron diseases and simplifies in vitro pain simulation. This advancement aids in verifying treatment efficacy and drug development, addressing the challenges of simulating pain externally and contributing significantly.
“Dynamics of axonal β-actin mRNA in live hippocampal neurons”
Byung Hun Lee, Seokyoung Bang, Seung-Ryeol Lee, Noo Li Jeon, Hye Yoon Park, Traffic, 23, 496-505 (2022)
“Comparison of the Efficacy of Optogenetic Stimulation of Glia versus Neurons in Myelination”
Kyuhwan Jung, Hong Nam Kim, Noo Li Jeon, Sujin Hyung, ACS Chemical Neuroscience, (2020)
“Optogenetic neuronal stimulation promotes axon outgrowth and myelination of motor neurons in a three‐dimensional motor neuron–Schwann cell coculture model on a microfluidic biochip”
Sujin Hyung, Seung‐Ryeol Lee, Yeon Jee Kim, Seokyoung Bang, Dongha Tahk, Jong‐Chul Park, Jun‐Kyo Francis Suh, Noo Li Jeon, Biotechnology and Bioengineering, 116 (10), 2425-2438 (2019)
“Modeling neural circuit, blood–brain barrier, and myelination on a microfluidic 96 well plate”
Seung-Ryeol Lee1, Sujin Hyung1, Seokyoung Bang1, Younggyun Lee, Jihoon Ko, Somin Lee, Hyung Joon Kim, Noo Li Jeon, Biofabrication, 11, 3 (2019)
Air-liquid interface model
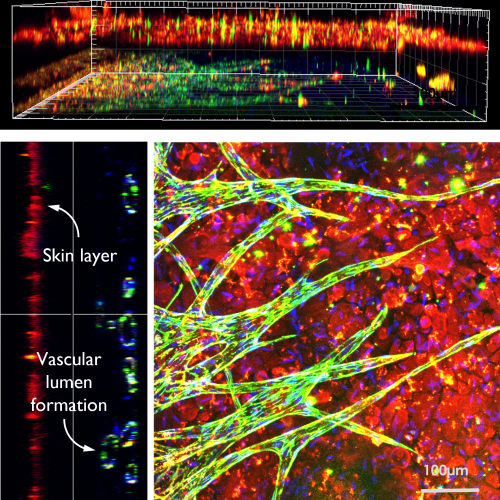
The air-liquid interface model in organ-on-chip technology enhances respiratory disease research and drug testing accuracy by exposing cells to both air and liquid, better mimicking respiratory tissues. Traditional 2D cell cultures fall short in replicating the human body’s complexity. This model is particularly beneficial for studying organs like the lungs and accurately simulates drug absorption, distribution, metabolism, and excretion. Our lab uses 3D printing to create complex structures forming epithelial layers at the air-liquid interface, developing multilayered models for organs such as the lungs, intestines, and skin. These ex vivo models closely mimic in vivo conditions, advancing high-throughput organ-on-chip modeling.
“Microfluidic approach for testing chemical irritants microvessels network”
Jihoon Ko, Soojung Oh, Noo Li Jeon, The 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2016), Ireland (Oct. 10, 2016)
“Engineering of in vitro 3D capillary bed in micro-pore-embedded microfluidics”
Soojung Oh, Hyun Ryul Ryu, Dongha Tahk, Jihoon Ko, Noo Li Jeon, The 8th International Conference on Microtechnologies in Medicine and Biology (MMB 2016), Korea (Apr. 22, 2016)
“Skin angiogenesis with keratinocytes on microfluidic”
Norhana Jusoh, Soojung Oh, Jin Young Lee, Hae Kwang Lee, Noo Li Jeon, The 8th International Conference on Microtechnologies in Medicine and Biology ( MMB 2016), Korea (Apr. 21, 2016)